Proyecto iroNKiller
Proyecto iroNKiller: Joining the forces of Natural Killer Cells and Ferroptosis to treat Refractory Neuroblastoma

Summary
Neuroblastoma (NB), a rare pediatric solid tumor, accounts for 15% of childhood cancer deaths. Immunotherapy has improved the survival for hematological malignancies, but solid tumors remain a challenge. The high heterogeneity, low mutational load, and strong immunosuppressive tumor microenvironment (i-TME) have hindered the success of immunotherapy in NB. Recently, natural killer (NK) cells have stood as a promising immunotherapeutic tool, as they don’t depend on specific mutations. Still, clinical trials for NB show only modest results, proving further action is needed to overcome the i-TME. Late findings show that refractory tumors often respond to Ferroptosis, a novel cell death mechanism that is highly immunogenic. Cancer cells undergoing ferroptosis release HMGB1 and other well-known recruiters of NK cells. Moreover, ferroptotic drugs can act through several mechanisms and can be adapted to patients with different tumor characteristics. The goal of IronKiller is to combine ferroptotic drugs with NK cell therapy to treat refractory NB, and to perform in silico modelling of the results to predict which patients would benefit from this novel therapy.
The fellow is an experienced biologist and engineer joining a clinical research group strong in immunotherapies. We will follow an in vitro-in vivo-in silico strategy that will cover a variety of disciplines, from basic biology and pre-clinical research, all the way to bioinformatics and machine learning. This project will reinforce the fellow’s experience in cell death mechanisms and translational research, and I provide new knowledge and skills in the areas of cell immunotherapy and oncological mathematical modelling. Along with the research work, the diverse training, management and communication activities planned, will play a key role in advancing the professional development of the fellow towards becoming an independent academic group leader in translational cancer research.
AIMS AND OBJECTIVES
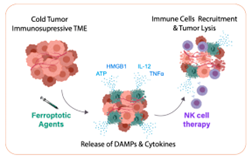
The goal of this project is to combine NK cell immunotherapy with ferroptotic agents to treat resistant HR-NB, and to perform mathematical modelling to predict which patients could benefit from this combination. This goal will be divided in four specific objectives:
- to characterize the immune response triggered by ferroptosis in HR-NB;
- to identify and validate at least one treatment combination;
- to generate a mathematical model that can assist with treatment selection for the highly heterogeneous NB.
- to undergo formal and informal training aimed to fully master this new knowledge and reach scientific maturity.
EXPECTED RESULTS AND IMPACT
IronKiller will take advantage of cutting-edge technology and models to transcend the state-of-the-art as follows:
- o Improve the understanding of the ferroptosis-induced immunogenic mechanisms. The immunogenicity caused by ferroptotic agents is fairly unexplored and completely unknown in the context of NB.
- o A treatment strategy combining ferroptosis-inducing drugs with immune cell therapy has never been explored before and presents a very high potential to overcome the i-TME.
- o NBs are extremely heterogeneous therefore, one-fits-all strategies are not appropriate. Combined flexible strategies are essential. This project will explore the combination of NK cells with a variety of ferroptotic agents and will do so in a cohort of models that aim to represent NB heterogeneity.
- o The mathematical modelling of the data obtained will help advance our ability to select personalized treatments based on tumor characteristics, which is especially challenging for the highly heterogeneous NB.
WHAT HAVE WE DONE SO FAR
We started the project by exploring, in an in vitro setting, if treatment with ferroptosis inducing compounds could enhance the infiltration and activity of Natural Killer (NK) cells to treat neuroblastoma.
- First, we have used four conventional neuroblastoma cells lines to identify if treatment with ferroptosis-inducing drugs could lead to the release of damage associated patterns (DAMPS) to attract NK cells. Preliminary results indicate there is a modified release of HMGB1. Although promising, further experiments are needed, as this effect seems to be dependent on the cells line, the drug, the mechanism of action, the dose and the time of treatment. This could open the door to personalized treatments.
- We also assessed the effect that these compounds have on NK cells and found that some ferroptosis inducing methods can be more damaging than others. Therefore, we conclude that careful selection and sequential treatment are important.
- Using four neuroblastoma organoid models derived from patient-derived xenograft models (PDX) we explored if pre-treatment with low dose ferroptosis-inducing compounds could enhance the infiltration of NK cells into the organoids. We tested multiple compounds and identified Erastin as the most successful one. Interestingly, it was also one of the ones that had a milder effect on NK cells.
- Using neuroblastoma PDX-derived organoids, we are in the process of exploring if this enhanced infiltration is due to an upregulation of NK ligands on the surface of neuroblastoma cells. To figure this out, we are currently performing RNA analysis of treated organoids and immunohistochemistry analysis on fixed pre-treated organoids.
- Additionally, because Erastin is not an ideal drug for in vivo work and it is not approved in humans, we are exploring two other compounds with the same mechanism of action, that are approved for their use in humans: Sorafenib and Sulfasalazine. If either of these is successful, this will accelerate the clinical implementation of this novel treatment.
We have also started exploring the ideal ways of combining NK cell treatment in and in vivo setting with other treatments, such as chemotherapy and ferroptosis-inducing drugs.
- Using two different PDX neuroblastoma models with different phenotypes, we have explored in vivo the capacity of NK cells to infiltrate the different models, the time they take to reach the tumor, and their efficacy on each model. This has allowed us to understand the behavior of NK cells in different neuroblastoma models and plan for combination treatments based on patient characteristics.
- Additionally, we have also explored the creation of new PDX and organoid models to expand our library. We have done so by implanting tumors from patients from our hospital and by establishing collaborations with other European partners, such as Lund University and the Hospital Sant Joan de Deu.
- Currently, a new experiment in vivo using PDX neuroblastoma models is being set to test the best combinations identified in vitro.
DISSEMINATION ACTIVITIES
Oral and poster presentations have been made in the following national and international seminars, symposiums and conferences:
- Up to 4 seminars so far given to the Clinical Unit of Translational Research in Pediatric Oncology, Hematopoietic Transplantation and Cell Therapy at IdiPAZ (2023-2025).
- Poster presentation at the international conference Advances in Neuroblastoma Research ANR2025 (upcoming 24-28/05/2025)
- Oral Presentation at the Alumni Symposium at the Lewis College of Science and Letters at Illinois Institute of Technology (upcoming 21-22/05/2025).
- Invited speaker presentation at the Workshop: From primary cells to organoids – the proliferative and developmental fate; organized by the University of Tunisia. (19/11/2024).
- Seminar at Lund University, to the group of Molecular Pediatric Oncology (05/11/24).
- Oral presentation at the weekly seminar organized by the Department of Biology at Illinois Institute of Technology (11/09/2024)
- Poster presentation at the international conference AACR Advances in Pediatric Cancer (05-06/09/2024)
- Seminar at the Hospital Sant Joan de Deu, to the Pediatric Cancer Research Group (19/07/2024).
- Seminar at the MOLAB group in Ciudad Real (11/10/2023).
OTHER ACTIVITIES OF INTEREST
Besides research, seminars and conferences, the fellow has engaged into other scientific-related activity, in the context of her MSCA fellowship. Some examples are:
- Leading the Neuroblastoma Work Group at the University Hospital of La Paz, which brings together oncologists, surgeons, pathologists, geneticists and researchers to discuss the latest advances in neuroblastoma treatment and how to implement them at our hospital.
- Volunteered at the European Researchers Night organized at the National Center of Oncological Research (CNIO).
- Become a member of SIOPEN, the European Society of Pediatric Cancer - Neuroblastoma Research Network.
- Performing student supervision of three bachelor students and two master students, as well as co-supervision of one PhD student.
- Member of a PhD Thesis defense committee, of the student Jara Martín at Hospital Sant Joan de Deu, Universidad de Barcelona.
- Collaboration on a publication about ferroptosis and neuroblastoma: PRDX6 contributes to selenocysteine metabolism and ferroptosis resistance. DOI: 10.1016/j.molcel.2024.10.027
- Established strong collaborations with patient associations and foundations such as the Neuroblastoma Foundation and CRIS Foundation.
IMPACT OF THE MSCA FELLOWSHIP ON THE FELLOW’S CAREER PATH
This project has deeply impacted the fellow’s career in multiple aspects. She has widened her knowledge on pediatric cancer, building a strong relationship with clinicians and learning how to improve translational research to reach the clinic faster. She has also received high-skilled training on how to work with immune cell therapies and use them in cancer research. Thanks to this project she has been able to establish new collaborations with experts in the field. And most importantly, with the support of her supervisor and institution, she has been awarded with a 5-year tenure track contract Miguel Servet through the Spanish National Health Institute Carlos III (ISCIII) and a 3-year national project on healthcare research (FIS) also from ISCIII.