|
Composition
|
Name
|
Position
|
Institution
|
|
Antonio Javier Carcas Sansuán
|
Jefe de Servicio
Profesor Titular. Facultad de Medicina
|
Hospital Universitario La Paz
Universidad Autónoma de Madrid
|
|
Pilar Ayllón García
|
Técnico de laboratorio
|
Universidad Autónoma de Madrid
|
|
Alberto Borobia Pérez
|
Facultativo Especialista de Área en Farmacología Clínica
|
Hospital Universitario La Paz
|
|
Raquel de Madariaga Castell
|
Enfermera. Coordinadora de Ensayos Clínicos
|
FIBHULP
|
|
Lucía Díaz García
|
Facultativo Especialista en Farmacología Clínica
|
Hospital Universitario La Paz
|
|
Jesús Frías Iniesta
|
Catedrático Emérito. Facultad de Medicina
|
Universidad Autónoma de Madrid
|
|
Blanca Duque Bascuñana
|
Responsable de calidad
|
Universidad Autónoma de Madrid
|
|
Alberto García Bartolomé
|
Investigador Postdoctoral
|
Hospital Universitario La Paz
|
|
Irene García García
|
Facultativo Especialista en Farmacología Clínica
|
Hospital Universitario La Paz
|
|
Andrea Guzmán de Antonio
|
Investigadora Predoctoral
|
FIBHULP
|
|
Cristina López Crespo
|
Enfermera. Coordinadora de Ensayos Clínicos
|
FIBHULP
|
|
Alicia Marín Candón
|
Medico Especialista en Farmacología Clínica
|
FIBHULP
|
|
José Carlos Martínez Ávila
|
Investigador Postdoctoral
|
Hospital Universitario La Paz
|
|
Lucía Martínez de Soto
|
Coordinadora de Ensayos Clínicos
|
FIBHULP
|
|
Vega Mauleón Martínez
|
Enfermera. Coordinadora de Ensayos Clínicos
|
FIBHULP
|
|
Pablo Molina Benítez
|
Investigador Predoctoral
|
FIBHULP
|
|
Paloma Moraga Alapont
|
Gestora de proyectos SCReN (Spanish Clinical Research Network)
|
FIBHULP
|
|
María Cristina Murano Murano
|
Investigador Postdoctoral
|
FIBHULP
|
|
Paula Prieto Correa
|
Monitora de Ensayos Clínicos
|
FIBHULP
|
|
Rocío Prieto Pérez
|
Gestora de proyectos SCReN (Spanish Clinical Research Network)
|
FIBHULP
|
|
Laura Vitón Vara
|
Enfermera. Coordinadora de Ensayos Clínicos
|
FIBHULP
|
|
Hoi Yan Tong
|
Responsable de Farmacovigilancia
|
Hospital Universitario La Paz
|
Strategic Objective
Clinical Pharmacology, as a scientific and professional discipline, aims to achieve the effective, efficient, and optimal use of medicines at both individual and collective levels. This means that Clinical Pharmacology is a cross-disciplinary field that must establish collaborations with numerous other disciplines and healthcare professionals.
In this regard, and within the field of clinical research, our research group focuses on two fundamental aspects:
1. Personalisation of pharmacological treatments: research and development of techniques and methods that enable the individualised use of medicines, improving efficacy and reducing risks.
2. Improvement of therapeutics through the generation of high-quality evidence on the efficacy of clinical trials, strategies for identifying, evaluating, and preventing adverse drug reactions (pharmacovigilance programmes), and assessing the appropriateness and effectiveness of drug use (drug utilisation studies).
MISSION
To promote and strengthen clinical research activities in therapeutic personalisation and clinical trials within the National Health System, contributing to the development of new treatments, the improvement of healthcare practice, and the health of citizens.
VISION
To establish ourselves as a leading research group in the development of high-quality clinical trials and in the innovation of clinical pharmacotherapeutic research.
VALUES
Quality
Innovation
Integrity and Respect
Commitment
GROUP EXPERIENCE
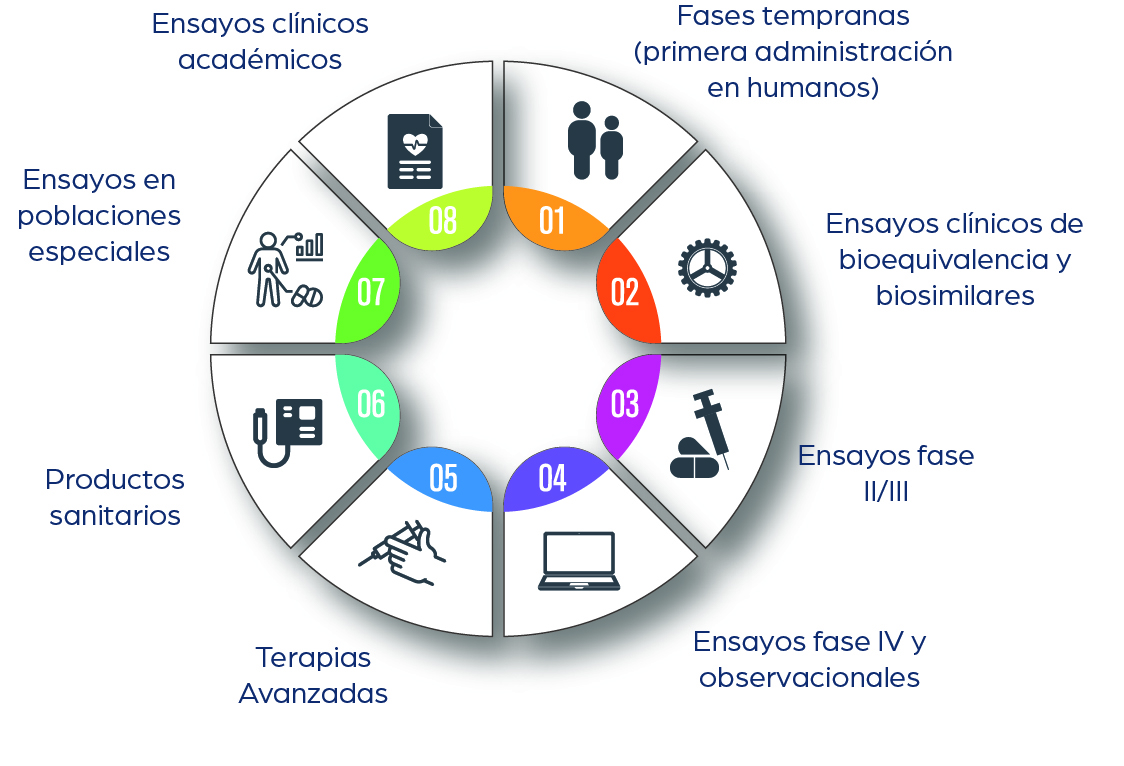
Clinical Trials
Pharmacotherapeutic Personalisation
Pharmacovigilance

Research Lines
The main research lines of this Research Group are:
1. Development of clinical research and clinical trials to generate evidence on the efficacy, safety, and proper use of human medicines through Phase I to IV clinical trials.
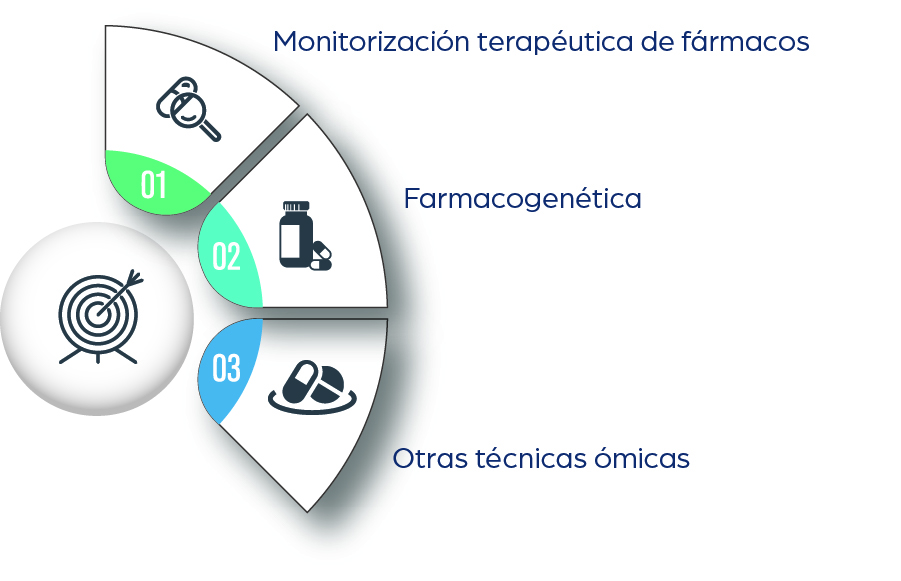
2. Personalisation and precision pharmacotherapy: research and development of techniques and methods that enable the individualised use of medicines, improving efficacy and reducing risks. This includes techniques such as Therapeutic Drug Monitoring (TDM), pharmacogenetics, and other approaches to precision pharmacotherapy.
Portfolio Services/Prices
|